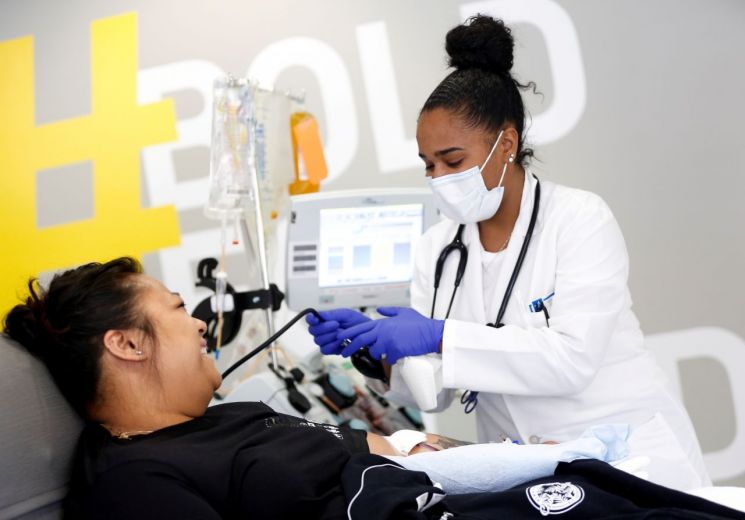
The U.S. Food and Drug Administration(FDA) has reportedly authorized the use of blood plasma from patients who have recovered from COVID-19 as a treatment for the illness.
According to Reuters, the FDA said on Saturday that early evidence suggests blood plasma can decrease mortality and improve the health of patients when administered in the first three days of their hospitalization.
The FDA reportedly said that it determined the safety of the product in an analysis of 20-thousand patients who received the treatment, adding that 70-thousand patients have been treated using blood plasma so far.
Reuters said the so-called “emergency use authorization” by the FDA came on the eve of the Republican National Convention, where Trump will be nominated to run for re-election.
SOURCE: KBS WORLD RADIO